Formulation Development
Our proprietary RACTAB technology
To answer the needs from medical professionals, Towa improves our value-added generics in response to the demands of medical professionals. An example of such a product is orally disintegrating (OD) tablets developed using our proprietary RACTAB technology.
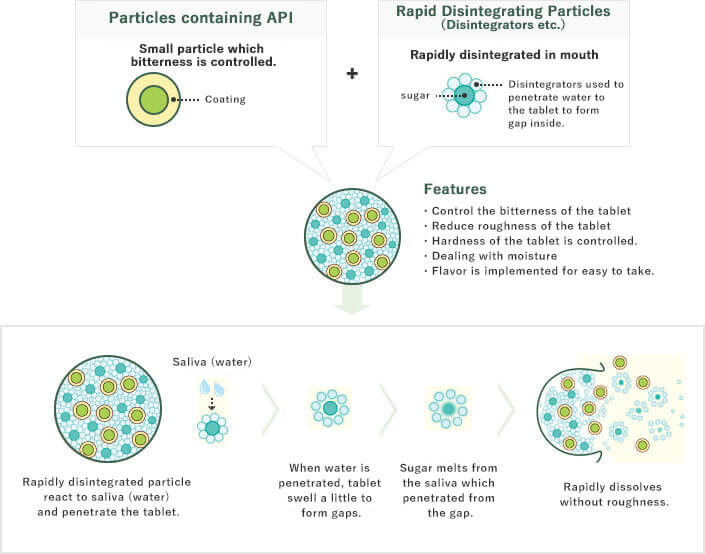
We want to provide products for patients with swallowing disorders (e.g., due to age, complications) or with limited water intake. In addition, we want to develop products that can be taken without water whenever necessary. To overcome the challenge, Towa has developed unique technology 『RACTAB(Rapid and Comfortable Tablets)technology』. Hardness of tablets is incompatible with easy disintegration. Towa has overcome this existing problem, and developed tablets with both easy disintegration and sufficient hardness. Towa is also committed to developing other value-added products using a variety of formulation technologies, including "masking technology" used to reduce bitterness.

『RACTAB technology』 Formulation Design
To manufacture an oral tablet, Active Pharmaceutical Ingredients (APIs) and excipients such as sugar and starch are compressed and solidified. Thus, with the ingredients closely bound together, the tablet dissolves in water slowly. On the other hand, ingredients of OD (orally disintegrating) tablets that can be taken without water are loosely bound together using a unique device and method, which allows water to easily penetrate the tablet, resulting quick disintegration. However, such tablets are friable and must be handled with more care than conventional tablets to prevent them from breakage. With the “RACTAB technology,” the particles in OD tablets are compressed and solidified in the same way as conventional tablets. However, we have ingeniously worked on these particles. Although the excipients of the tablet are closely bound together, our original rapid disintegrating particles allow a small amount of water to loosen and penetrate them easily. In this way, we can manufacture OD tablets that can be handled in the same way as conventional tablets and yet dissolve quickly only with saliva. Also, we can manufacture OD tablets that are not bitter even after they dissolve in the mouth by mixing small granules (functional API particles) that encapsulate bitterness and rapidly disintegrating particles.
Other Improvements
For promoting correct use of drugs, creation of drugs that are easily taken
-
Adding new formulation
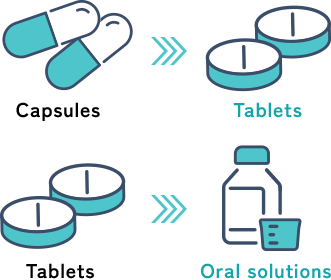
We try to change the formulation from capsules to tablets and from tablets to oral solutions so that drugs can be taken easily.
-
Smaller tablets

Large tablets are hard to swallow. On the other hand, tablets that are too small are difficult to handle. The preferred size of a tablet is generally considered to be between 7 mm and 8 mm. We develop smaller tablets that are easy to handle and swallow.
-
Masking technology*

Since many of the APIs bitter, with our “masking technology” we coat the API , so that the bitterness is hardly noticeable. We also add flavor to further help patients take medication with ease.
However, even if we try to apply a coating, if their shape is distorted, they will not coat evenly resulting in bitterness in the mouth before swallowing. Towa is therefore conducting research on sphere-shape API that can be coated more evenly. -
Micro-granulation

Making small spherical particles reduce the roughness of the tablets that a patient could feel in the mouth.
*Masking technology
-
API scanned by electronic microscope.
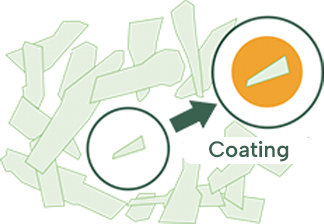
Coating become uneven if the particle of API is distorted.
-
API after improvement
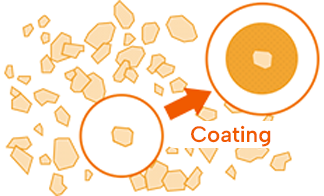
By forming the API particle in sphere, coating can be evenly performed resulting in reducing the bitterness of API.
Efforts toward easier-to-take tablets for patients and easier-to-handle tablets for medical professionals
-
Tablet printing

In 2013, we launched OD tablets with an imprint of the product name avoiding the score line for the first time in the world, allowing the product name to be read easily even after scoring. We believe this will help patients prevent medication errors and medical professionals can easily identify the tablet.
We introduced 2 color printing technology for better identification. We are pioneers in Japan for such technology. -
Photo-stability

Special coating are implemented to prevent the discoloration and degradation of tablets by light, and prevention from light packaging materials have been used.
-
Stability against moisture
We are also working on preventing deterioration in quality due to humidity when patients store at home.
