Project Story



Under this belief, we discovered through a thorough investigation process that one of the causes of nitrosoamine contamination, which poses a carcinogenic risk in pharmaceuticals, is trace amounts of nitrogen oxides (NOx) in the air. To uphold the ideal "Towa Quality," we have successfully utilized science to achieve manufacturing in an unprecedented environment of 1 part per billion (ppb) level of NOx, thereby controlling nitrosoamines. Our uncompromising challenge has no end.

The European Medicines Agency (EMA) pointed out that "sartan-based drugs (antihypertensives) were contaminated with N-nitrosodimethylamine (NDMA) and N-nitrosodiethylamine (NDEA), which are of carcinogenic concern," causing a worldwide shock.
EMA released guidance to avoid the contamination of nitrosoamine impurities. "NDMA was also detected in ranitidine (an acid suppressant)," leading pharmaceutical companies in Japan to conduct recalls. The Health Sciences Authority (HSA) of Singapore reported that "NDMA was detected in metformin products (a diabetes treatment drug), and a voluntary recall of some affected products was initiated." The Food and Drug Administration (FDA) also began an investigation.
In the United States, NDMA contamination in ranitidine followed by nizatidine known as a gastric acid inhibitor and metformin products was reported; New nitrosoamines impurities findings have been extended to rifampicin and rifapentine, leading to industry-wide voluntary recall; FDA issued guidance for industry to perform nitrosoamine risk assessments in drugs.
With further expansion of the contamination of small-molecule nitrosoamines impurities represented by NDMA, the discovery of nitrosoamines drug substance-related impurities (NDSRIs = Nitrosoamine Drug Substance-Related Impurities) in which the drug substance itself has been nitrosated was recognized as a worldwide issue.
The Ministry of Health, Labour and Welfare (MHLW) issued a Notice of Voluntary Inspection, requiring pharmaceutical companies to "conduct risk assessments (until April 30, 2023) " and "implement and report on risk mitigation measures (until October 31, 2024)".
We concluded that dimethylamine, which remained in trace amounts in the metformin drug substance, was likely to react with atmospheric NOx to form NDMA and led to contamination during the granulation process and reported to MHLW.
We launched a cassette-screening analytical method covering nine regulated nitrosoamines so-called "Towa Amine Approach" to initiate a comprehensive nitrosoamines contamination risk assessment for all products.
Guided by Prof. Norimichi Takenaka at Osaka Metropolitan University, we installed NOx monitors to measure atmospheric NOx concentration in TOWA Osaka plant, and demonstrated a clear correlation between AUC of atmospheric NOx concentration and NDMA contamination in the product.
We achieved a world-first discovery by using model system to reveal that atmospheric NOx directly contributed to NDMA production during granulation/drying processes exposed to large amount of the air.
Meanwhile, group company Daichikasei launched contract nitrosoamine analysis services.
http://www.daichikasei.com/
FDA issued its final guidance on acceptable daily intake for NDSRIs, while Japan's MHLW notified to extend the deadline for implementation of risk mitigation measures from October 31, 2024 to August 1, 2025.
We disclosed actual data and its analysis to the MHLW, reporting that atmospheric NOx is a key driver for nitrosation during manufacturing process, and published our findings in ACS Organic Process Research & Development. 1) The study—showing that even tens of ppb NOx can contribute to generating nitrosoamines—received an enthusiastic response from all over the world. At the same time, we started an unprecedented study to reduce atmospheric NOx to the ppb level (one part in a billion) during manufacturing process, aiming to completely suppress nitrosoamines in the products. 1) Shohei Fukuda, Kanako Kondo, Shoji Fukumoto,* Norimichi Takenaka, Osamu Uchikawa and Itsuro Yoshida:Organic Process Research & Development.2023;27(11):2123-2133

The FDA released its final guidance to control nitrosoamine impurities, along with recommendations for mitigation strategies on both small molecule nitrosoamines and NDSRIs.
We developed the NOx adsorption filter system to reduce atmospheric NOx to the ppb range, and the findings paved the way for plant-wide deployment. The article was posted in ACS Organic Process Research & Development.2) 2) Ryota Nomura*, Takahiko Taniguchi, Kazuya Suzuoki, Rintaro Yokoi, Kanenao Akasaki, Kyoko Hirai, and Osamu Uchikawa:Organic Process Research & Development.2025;29(1):66-70

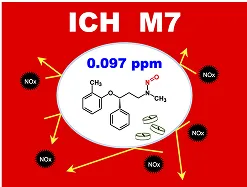
We submitted a paper to ACS Organic Process Research & Development 3) describing the achievement of a nitroso-atomoxetine content of 0.097 ppm in a seamless pilot manufacture of atomoxetine under NOx-free conditions by using a NOx adsorption filter system.
Now we are ready to implement next generation NOx adsorption filters across production lines.3) Ryota Nomura*, Takahiko Taniguchi, Koji Kitada, Mamoru Otsuki, Takuma Tamai, Kazuya Suzuoki, Rintaro Yokoi, Kanako Kondo, and Osamu Uchikawa:Organic Process Research & Development.2025;29 (9):2259–2264
Please feel free to contact us
if you have any questions or would like to discuss our initiatives.
—our team will be happy to assist you.
